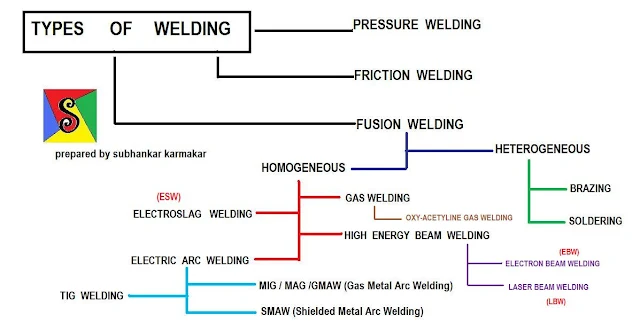
Fundamentals of metals
There are two main forms of solid substance, characterizing different atoms arrangement in their microstructures:
Amorphous solid
Crystalline solid
Amorphous solid
Amorphous solid substance does not possess long-range order of atoms positions. Some liquids when cooled become more and more viscous and then rigid, retaining random atom characteristic distribution.
This state is called undercooled liquid or amorphous solid. Common glass, most of Polymers, glues and some of Ceramics are amorphous solids. Some of the Metals may be prepared in amorphous solid form by rapid cooling from molten state.
Crystalline solid
Crystalline solid substance is characterized by atoms arranged in a regular pattern, extending in all three dimensions. The crystalline structure is described in terms of crystal lattice, which is a lattice with atoms or ions attached to the lattice points. The smallest possible part of crystal lattice, determining the structure, is called primitive unit cell.
Examples of typical crystal lattice are presented in the picture:
Metal crystal structure and specific metal properties are determined by metallic bonding – force, holding together the atoms of a metal. Each of the atoms of the metal contributes its valence electrons to the crystal lattice, forming an electron cloud or electron “gas”, surrounding positive metal ions. These free electrons belong to the whole metal crystal.
Ability of the valence free electrons to travel throughout the solid explains both the high electrical conductivity and thermal conductivity of metals.
Other specific metal features are: luster or shine of their surface (when polished), their malleability (ability to be hammered) and ductility (ability to be drawn).
These properties are also associated with the metallic bonding and presence of free electrons in the crystal lattice.
The following elements are common metals:
aluminum(Al), barium(Ba), beryllium(Be), bismuth(Bi), cadmium(Cd), calcium(Ca), cerium(Ce), cesium(Cs), chromium(Cr), cobalt(Co), copper(Cu), gold(Au), indium(In), iridium(Ir), iron(Fe), lead(Pb), lithium(Li), magnesium(Mg), manganese(Mn), mercury(Hg), molybdenum(Mo), nickel(Ni), osmium(Os), palladium(Pd), platinum(Pt), potassium(K), radium(Ra), rhodium(Rh), silver(Ag), sodium(Na), tantalum(Ta), thallium(Tl), thorium(Th), tin(Sn), titanium(Ti), tungsten(W), uranium(U), vanadium(V), zinc(Zn).
Amorphous solid
Crystalline solid
Amorphous solid
Amorphous solid substance does not possess long-range order of atoms positions. Some liquids when cooled become more and more viscous and then rigid, retaining random atom characteristic distribution.
This state is called undercooled liquid or amorphous solid. Common glass, most of Polymers, glues and some of Ceramics are amorphous solids. Some of the Metals may be prepared in amorphous solid form by rapid cooling from molten state.
Crystalline solid
Crystalline solid substance is characterized by atoms arranged in a regular pattern, extending in all three dimensions. The crystalline structure is described in terms of crystal lattice, which is a lattice with atoms or ions attached to the lattice points. The smallest possible part of crystal lattice, determining the structure, is called primitive unit cell.
Examples of typical crystal lattice are presented in the picture:
Metal crystal structure and specific metal properties are determined by metallic bonding – force, holding together the atoms of a metal. Each of the atoms of the metal contributes its valence electrons to the crystal lattice, forming an electron cloud or electron “gas”, surrounding positive metal ions. These free electrons belong to the whole metal crystal.
Ability of the valence free electrons to travel throughout the solid explains both the high electrical conductivity and thermal conductivity of metals.
Other specific metal features are: luster or shine of their surface (when polished), their malleability (ability to be hammered) and ductility (ability to be drawn).
These properties are also associated with the metallic bonding and presence of free electrons in the crystal lattice.
The following elements are common metals:
aluminum(Al), barium(Ba), beryllium(Be), bismuth(Bi), cadmium(Cd), calcium(Ca), cerium(Ce), cesium(Cs), chromium(Cr), cobalt(Co), copper(Cu), gold(Au), indium(In), iridium(Ir), iron(Fe), lead(Pb), lithium(Li), magnesium(Mg), manganese(Mn), mercury(Hg), molybdenum(Mo), nickel(Ni), osmium(Os), palladium(Pd), platinum(Pt), potassium(K), radium(Ra), rhodium(Rh), silver(Ag), sodium(Na), tantalum(Ta), thallium(Tl), thorium(Th), tin(Sn), titanium(Ti), tungsten(W), uranium(U), vanadium(V), zinc(Zn).