Tuesday, 22 September 2020
MOVING COIL GALVANOMETER
TORQUE EXPERIENCED BY A CURRENT LOOP IN A UNIFORM MAGNETIC FIELD
Monday, 21 September 2020
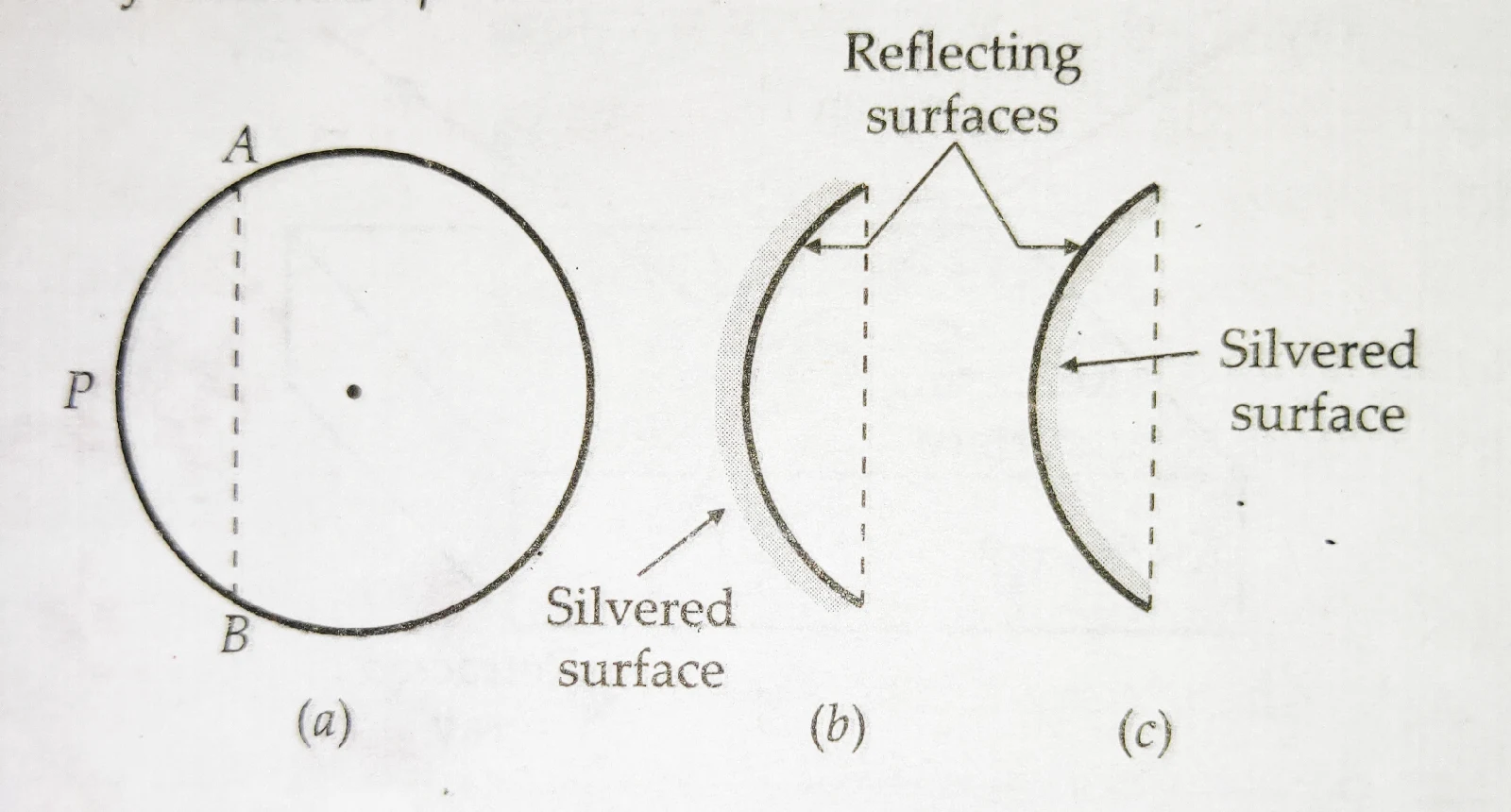
Lecture- 5 : CLASS-X: SCIENCE : Chapter: REFLECTION OF LIGHT & NUMERICALS
Sunday, 20 September 2020
Lecture 1: CLASS XI : PHYSICS : CHAPTER - 5 : LAWS OF MOTION
Wednesday, 16 September 2020
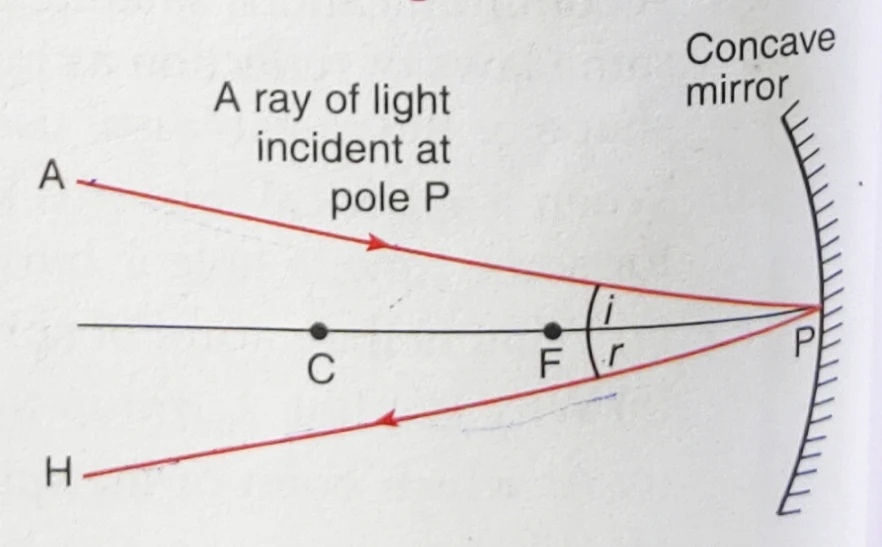
Lecture- 6 : CLASS-X: SCIENCE : Chapter: REFLECTION OF LIGHT & SIGN CONVENTIONS
CLASS X | SCIENCE | LIGHT
Tuesday, 15 September 2020
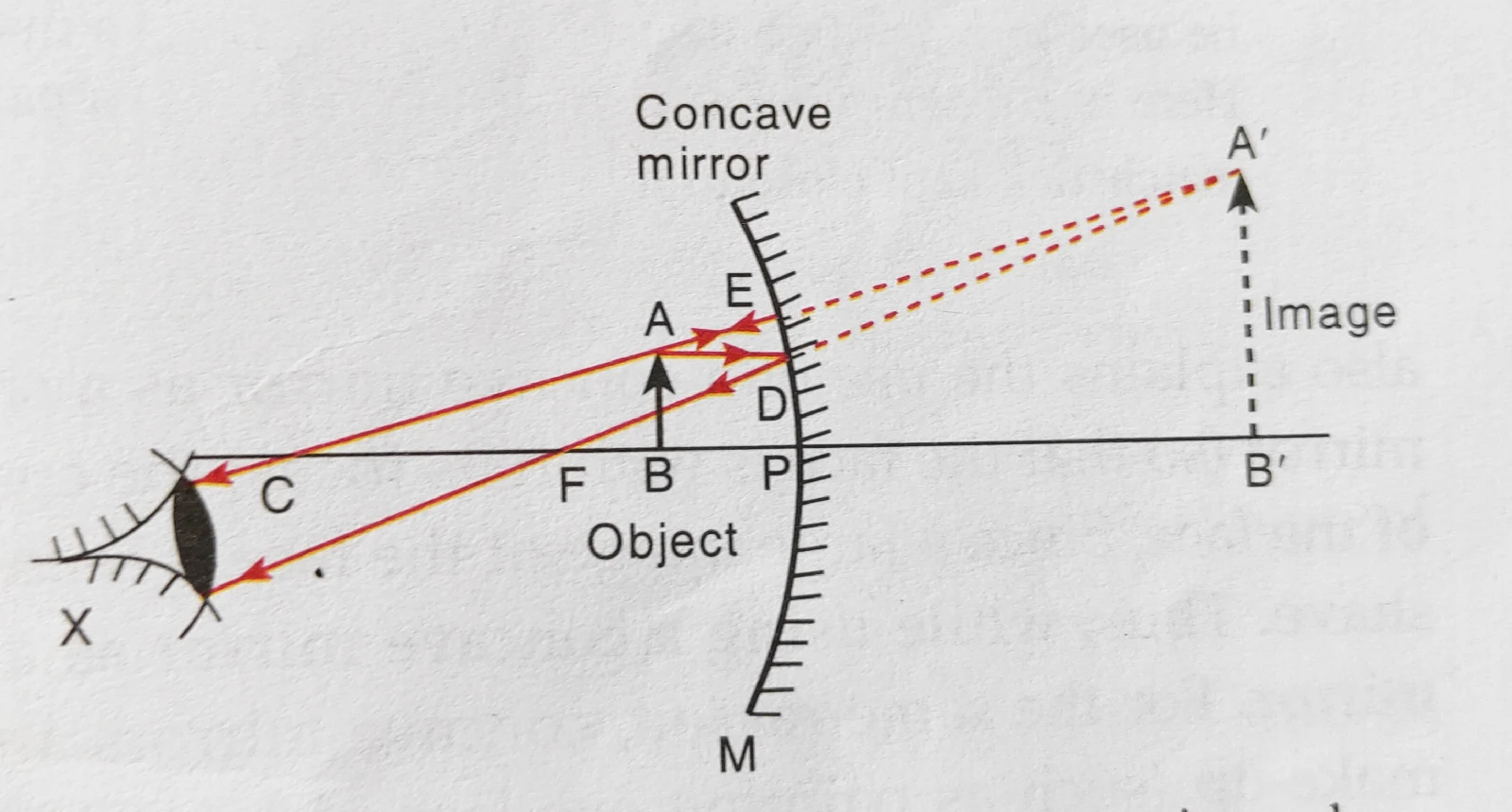
Lecture- 5 : CLASS-X: SCIENCE : Chapter: Reflection of light & concave mirror
CLASS X | SCIENCE | LIGHT
Sunday, 13 September 2020
LECTURE -2 : CLASS VIII : SCIENCE : CHAPTER 4 : MATERIALS : METALS & NON-METALS
CLASS VIII |
SCIENCE | CHAPTER 4
Notes prepared by Subhankar Karmakar
CHEMICAL PROPERTIES OF METALS & NON METALS:
REACTION
OF METALS:
The
basic metal oxides turn red litmus to blue.
*(Metals
and R & B)
∴ Metal + Oxygen (from air) = Metal Oxide (basic oxide)
Magnesium burning in air:
I. When Magnesium (Mg) burns in air, it combines with the oxygen (O₂) of air to form magnesium oxide.
• Mg
+ O₂ = MgO (a basic oxide)
II. Magnesium oxide dissolves partially in water to form magnesium hydroxide Mg(OH)₂ solution:
• MgO
+ H₂O = Mg(OH)₂ (a base)
Sodium (Na) reacts with Oxygen in air and produces Sodium Oxide (Na₂O)
• Na
+ O₂ = Na₂O (a basic oxide)
Water
solution of Sodium Oxide forms Sodium Hydroxide (NaOH)
•
Na₂O + H₂O = NaOH
During
the rusting of iron, iron (Fe) metal combines slowly with the
oxygen (O₂) of air in the presence of water or moisture to form a
compound called iron oxide (Fe₂O₃). This iron
oxide is called rust. Damp air contains Oxygen (O₂) +
water (H₂O).
• Iron (Fe) + Oxygen (O₂) + water (H₂O) ⟹ Iron Oxide or rust (Fe₂O₃) (basic oxide)
•
Reaction of copper metal with moist air:
When
a copper object is exposed to moist air for a long time, then copper (Cu) reacts
with water (H₂O), carbon dioxide (CO₂) and oxygen (O₂) present in
moist air to form a green coating on the copper object. The green coating is a
mixture of copper hydroxide [Cu(OH)₂] and copper carbonate (CuCO₃) which
is formed by the action of moist air on copper object.
• 2Cu + H₂O + CO₂ + O₂ = Cu(OH)₂ + CuCO₃
•
Corrosion of copper: The formation of green coating of basic copper carbonate on the
surface of copper objects on exposure to moist air is called corrosion of
copper.
b.
Reactions of metal with water:
Metal + water = Metal
hydroxide + Hydrogen
Not all metals react with water. Some of the metals reacts with cold water, whereas some metals reacts with hot water and steam. It depends upon reactivity of metals.
Sodium and potassium very quickly reacts with cold water.
·
Magnesium reacts slowly with cold water and quickly with hot water
and zinc and iron slowly react with steam.
·
Sodium (Na) + water
(H₂O) → Sodium Hydroxide (NaOH) + Hydrogen (H₂)
· Sodium (Na) is a very reactive metal. It reacts with moisture, oxygen and other gases present in air. So, if sodium metal is kept exposed to air, it will react with the various components of air and get spoiled. In order to prevent its reaction with the moisture and other gases of air, sodium metal is always told under kerosene. Potassium metal is also very reactive and also kept in kerosene.
c. Reactions of metals with acids:
Most
of the metals react with dilute acids to form salts and hydrogen gas.
Metal + Acid → Salt +
Hydrogen gas.
Only less reactive metals like Copper, silver and gold do not react with dilute acids.
•
Magnesium reacts with dilute hydrochloric acid to form magnesium chloride
(salt) and hydrogen gas.
Magnesium + hydrochloric
acid → magnesium chloride + hydrogen gas
Mg + HCl → MgCl₂ + H₂
d. Reactions of metal with bases:
Only some metals react with bases to form salts and hydrogen gas. Like aluminium is a metal and Sodium hydroxide is a base. When aluminium is heated with sodium hydroxide solution, then sodium aluminate which is a salt and hydrogen gas is formed.
Sodium hydroxide +
aluminium → sodium aluminate + hydrogen
NaOH + Al → NaAlO₂ +
H₂
Zinc also reacts with bases like sodium hydroxide to produce hydrogen gas.
REACTION OF NON METALS:
a.
Reaction of nonmetals with oxygen:
Non metal + oxygen → non
metal oxide
Sulphur
+ oxygen → sulphur dioxide
S + O₂ → SO₂
Sulphur
dioxide dissolves in water to form sulphurous acid solution
SO₂ + H₂O → H₂SO₃
b.
Reactions of nonmetals with water:
Non
metal oxides are acidic in nature and turn blue litmus to red.
The arrangement of metals in a vertical column in the order of decreasing reactivities is called the reactivity series of metals.
In reactivity series, the most reactive metal is placed at the top whereas the least reactive metal is placed at the bottom.
Potassium
is the most reactive metal, so it has been placed at the top of the reactivity
series. Gold is the least reactive metal so it has been placed at the bottom of
the reactivity series.
Potassium
(K) (most reactive)
Sodium
(Na)
Calcium
(Ca)
Magnesium
(Mg)
Aluminium
(Al)
Zinc
(Zn)
Iron
(Fe)
Lead
(Pb)
Copper
(Cu)
Silver
(Ag)
Gold
(Au) (least reactive)
Reactivity
of the metals decreases as we go down in the above series.