The case of distilled water, tap water, sea water and rainwater
1. Distilled water is non conductors of electricity
2. When some salts are dissolved in distilled water, it becomes a good conductor of electricity
Q. How can we make distilled water a conductor of electricity?
Ans. We can make distilled water a conductor by adding
(i) salts (ii) acids (iii) bases
Tap water:
As tap water contains small amount of various salts dissolved in it, it is a conductor of electricity.
We should never operate an electric appliances like heater, iron, mixer, grinder etc with wet hands nor we should touch electric switches.
In case of a fire, before fireman use big water hoses to throw water on a burning houses, the electric supply to that home is cut off first.
Drinking water:
Drinking water contains some amount of salts in it hence it is also a conductor of electricity.
Sea Water: Sea water is salty, it means a large amount of salts are dissolved in water, so they are very good conductor of electricity.
Rain water:
Due to the presence of small amount of acids in rain water, it is also a conductor of electricity.
Which one is better conductor drinking water or sea water and why?
Sea water contains more salts than that of drinking water, so sea water is a better conductor.
Q. Is it safe to carry out electric repairs during a rainy day?
Ans: As rain water is a good conductors of electricity, it is not safe for an electrician to carry out electric repair during a rainy day
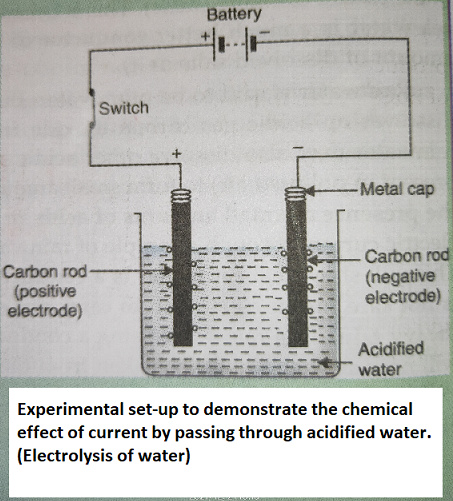
Chemical effects of electric current
When electric current is passed through acidified water by using carbon electrodes, then there is a chemical reaction takes place and Hydrogen and Oxygen will be released as water will be decomposed into hydrogen and oxygen.
ELECTROLYSIS
The chemical decomposition produced by passing electric current through a conducting liquid is called electrolysis. For example, When electric current is passed through acidified water by using carbon electrodes, then there is a chemical reaction takes place and Hydrogen and Oxygen will be released as water will be decomposed into hydrogen and oxygen.
Take two carbon rods and cleaning their metal caps they are fitted with wires and connected to a battery with a switch on the positive side. The carbon rods will act as electrodes, one is cathode (negative electrode) and anode (positive electrode). Take 250 ml of acidified water in the beaker and emerge the carbon rods into them. When we close the switch, electric current will pass through the acidified water and bubbles of gases will be produced at the two electrodes. The formation of gas bubbles at the two electrodes shows that a chemical change or chemical reaction takes place. This is a description of an activity which shows the chemical effects of electric current.
"If electric current is passed through acidified water, then bubbles of oxygen gas and hydrogen gas are produced at the two electrodes."
1. Oxygen will be produced at positive electrode (anode)
2. Hydrogen will be produced at negative electrode (cathode)
The fresh fruits and vegetables conduct electricity to some extent due to the presence of various salt solution in them.
Describe an activity to demonstrate the change in colour caused by the chemical effect of electric current.
We cut a potato into two halves. Taking one piece of cut potato and inserting to iron nails into it a little distance apart from one another. The iron nails are the two electrodes in this case. We connect the two terminals of a battery to the two iron nails by including a compass and a switch in the circuit as shown in the figure. When we pass the electric current through cut potato piece by closing the switch, we observe a deflection in the compass needle which indicates that potato is conducting electricity to some extent. Latest continue to pass electric current through water two piece for about half an hour, we shall notice a greenish blue spot on the cut surface of potato around the iron nail which is connected to the positive terminal of the battery. There is however no coloured spot around the other name which is connected to the negative terminal of the battery. The formation of a greenish blue spot around the positive electrode inserted in the surface of a cut potato shows that the chemical effect of current can bring about change in the colour of a conducting solution.
Applications of the chemical effect of electric current
1. Electroplating metals
2. Purification of Metals
3. Production of certain metals from their ores
4. Production of chemical compounds
5. Decomposing chemical compounds
Electroplating of metals
The process of depositing a thin a layer of a desired metal over a metal object with the help of electric current is called Electroplating.
The metal objects like taps, utencils, jewelry and other objects are electroplated with chromium, tin, nickel, silver, gold or copper.
Activity for electroplating:
How does copper can be electroplated on the surface of an object made of iron?
We shall take a beaker and pour copper sulphate solution in it. Now we take a copper plate and connected to the positive terminal of the battery with a switch. Again we take iron object like an iron key and connect it to the negative terminal of the battery. Therefore, copper plate and iron key will act as electrodes. Now when we close the switch , electric current will flow through the copper solution and copper will be diposited on the surface of the iron key. Thus the iron key will be electroplated with copper. During the copper plating of an iron key, coppee metal transferred from copper plate to the iron key through the copper sulphate solution. This process is also known as copper plating.
The following points should be remembered while electroplating:
1) The metal object on which electroplating is to be done is made the negative electrode or cathode. Therefore it must be connected to the negative terminal of the battery.
2) The metal whose layer is to be deposited is made the positive electrode or anode. It is connected to the positive terminal of the battery.
3) A water soluble salt of the metal to be deposited is taken as the electrolyte the electrolyte contains the metal to be deposited in the form of a soluble salt. For example, copper plating, we need copper sulphate solution.
Uses of electroplating:
1. Electroplating is used to cover iron and steel objects with a thin layer of chromium metal. This chromium layer gives an attractive shiny surface and also protects iron and steel objects from rusting. For example, bicycle handlebars, bicycle bells wheel rims, bathroom fittings LPG stoves motorcycle parts are often electroplated with chromium.
2. The less reactive and shiny metals like chromium, tin and nickel are electroplated on more reactive and dull looking metals like iron and Steel to protect them from corrosion and give them an attractive finish.
3. Electroplating is used to give objects made of metal a coating of more expensive metal to make them look more attractive. For example, less expensive metals are electroplated with more expensive metals like silver and gold to make jewellery.
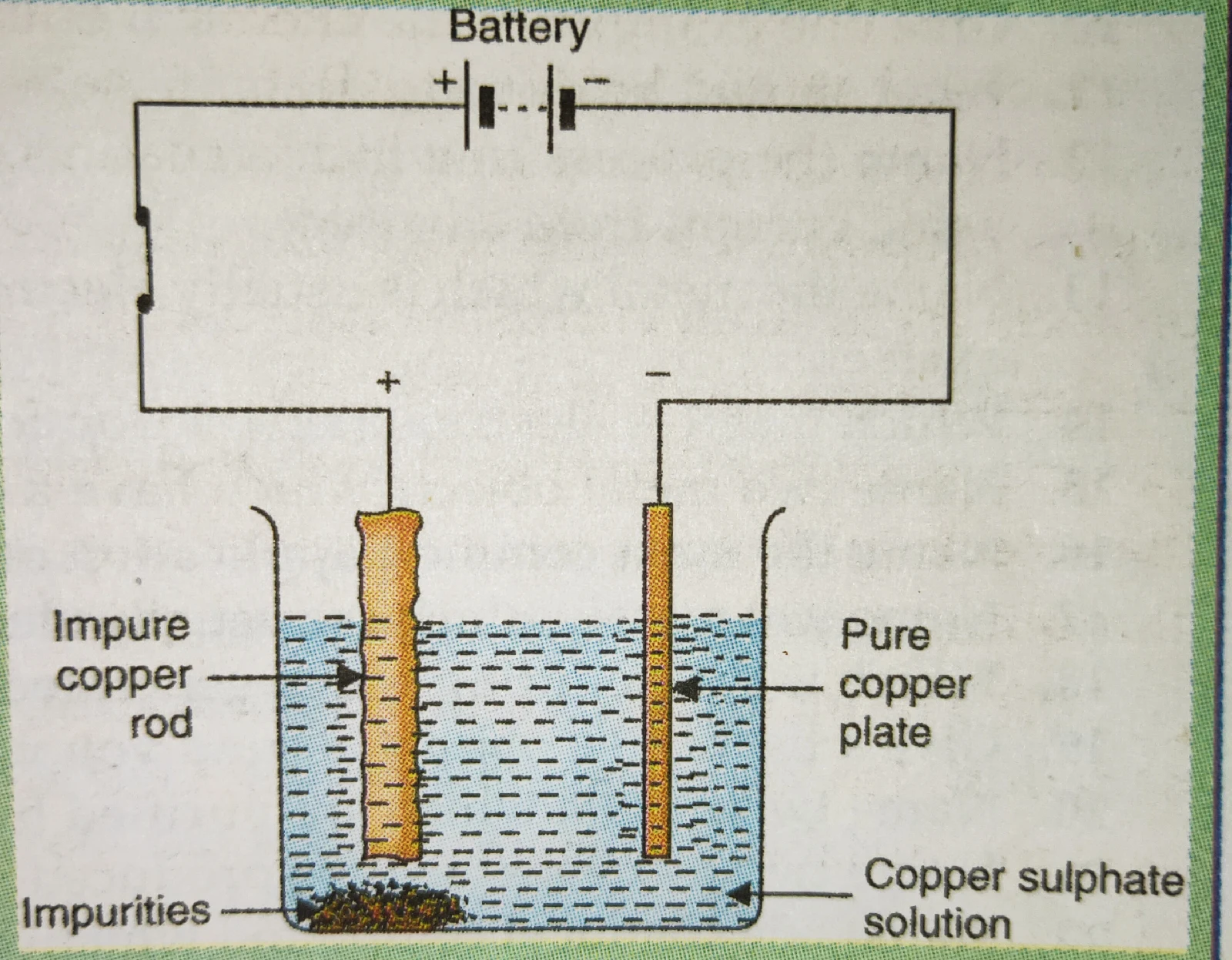
Purification of metals:
The chemical effect of electric current is also used in the purification of impure metals.
Impure metals are collected from their naturally occurring compounds called ores. In the purification of an impure metal by using the chemical effect of electric current or electrolysis,
a. Thick rod of a metal is made positive electrode or anode and it is connected to the positive terminal of the battery.
b. A thin strip of pure metal is made negative electrode or cathode and it is connected to the negative terminal of the battery.
c. A water soluble salt of the metal to be purified is taken as electrolyte.
Take 250 ml of distilled water in a clean beaker. Dissolve two teaspoonfuls of copper sulphate in it. Add a few drops of dilute sulphuric acid to copper sulphate solution. A thick rod of a copper metal is made positive electrode by connecting it to the positive terminal of the battery. 18 plate of pure copper metal is made negative electrode or cathode by connecting it to the negative terminal of the battery. Switch on the electric current by closing the switch. Allow the current to pass for about half an hour. It will be observed that the A copper rod goes on becoming thinner and thinner where is the pure copper plate goes on becoming thicker and thicker. This is because the A copper metal of Android goes on dissolving in copper sulphate solution where is the pure metal from copper sulphate solution goes on depositing on copper plate cathode. Impurities present in a rod of copper fall to the bottom of the beaker.
Production of metals:
The chemical effect of electric current is used in the production or extraction of certain metals from their naturally occurring compounds called ores.
Production of compounds:
The chemical effect of electric current or electrolysis is used in the production of various chemical compounds like sodium hydroxide is produced by the electrolysis of an aqua solution of sodium chloride.
Decomposition of compounds:
The chemical effect of electric current or electrolysis is used to decompose various chemical compounds into their elements.





No comments:
Post a Comment